Files
Download Full Text (876 KB)
Abstract
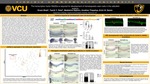
Hematopoietic Stem Cells (HSCs) are the self-renewing population of cells that generate all erythrocytes and leukocytes over the lifetime of a vertebrate organism. HSCs are also the therapeutic units of curative bone marrow transplants used in the treatment of blood malignancies and in gene therapy for genetic blood disorders. In all vertebrate embryos, HSCs originate from the floor of the embryonic dorsal aorta during the endothelial to hematopoietic transition. Nascent HSCs will bud into the blood vessel and be carried to maturation sites by the embryonic blood flow. Despite the curative potential of HSC transplants in blood disorders, this approach is limited by low numbers of immunologically compatible HSCs for transplantation. A major objective is to generate unlimited numbers of patient matched HSCs from patient derived induced pluripotent stem cells (iPSCs) to alleviate this challenge to therapy, however it has not yet been possible to generate HSCs from iPSCs. This is likely because key developmental signals remain unknown. This makes the study of HSC development in the vertebrate embryo a key area of biomedical study. Previous work has suggested glucose metabolism generates reactive oxygen species (ROS) which are needed for HSC development. Nfe2l2a is a transcription factor and master regulator of the cellular antioxidant response and is activated by increased levels of ROS in the cell. Nfe2l2a has been previously indicated as a regulator of ROS mediated cytokine signaling in adult HSCs. Additionally, nfe2l2a is expressed in the embryonic vasculature of zebrafish embryos, which is the origin of HSCs. We sought to determine whether nfe2l2a is required for the development of HSCs in the zebrafish embryo. The zebrafish pre-clinical animal model serves as an appropriate model due to evolutionary conservation of hematopoiesis between the zebrafish and humans. We examined the expression of the conserved hematopoietic markers runx1, cmyb, and rag1 in embryos injected with an antisense morpholino oligonucleotide which disrupts nfe2l2a mRNA translation. We observed a significant reduction in the expression of these markers, indicative of impaired HSC development. Since normal patterning of the embryonic dorsal aorta is required for HSC development, the embryonic vasculature of nfe2l2a morphants was also examined for expression of key marker genes, which indicated normal patterning of these tissues. Interestingly, examination of fli1:EGFP transgenic embryos injected with nfe2l2a morpholino revealed arterio-venous malformations which are indicative of defects in the segregation of the dorsal aorta and cardinal vein. Overall, our work has revealed that nfe2l2a activity is required for the development of HSCs in the zebrafish embryo, possibly by controlling segregation of the dorsal aorta and cardinal vein blood vessels during development. Future experiments will be aimed at determining how nfe2l2a regulates this process.
Publication Date
2022
Subject Major(s)
Biology
Keywords
zebrafish, Nfe2l2a, hematopoietic stem cell, embryo, transcription factor, vasculature, knockdown, development, morpholino
Disciplines
Developmental Biology
Current Academic Year
Junior
Faculty Advisor/Mentor
Dr. Erich Damm
Rights
© The Author(s)
Recommended Citation
We hope that the citation will be in APA format.